広範なヒト疾患に関わるタンパク質架橋酵素(Transglutaminase: TGase)の役割を理解するためには、各TGaseアイソザイム(哺乳類において8種存在)のmRNAとタンパク質の発現量や局在の理解はもとより、細胞や組織レベルでの活性化量および活性局在性を把握すること、各TGaseアイソザイムにより架橋される標的基質タンパク質を網羅的に理解することが重要である。
私たちはこれまでに、各TGaseアイソザイム選択的な基質となり架橋されるGln残基を含むペプチド配列を同定し、これらのペプチドおよびLys残基を模倣した標識一級アミンを用いて、各種生理的現象(細胞分化や皮膚形成)、病態組織(肝・腎・肺の細胞死や炎症、線維化)において、各TGaseアイソザイムの活性化の程度や局在、基質タンパク質の同定を行ってきた。
In order to understand the role of protein crosslinking enzyme (TGases) in a wide range of human diseases,
it is nessesary to evaluate the expression and localization of each TGase isozyme (there are eight species in mammals) in mRNA and protein levels as well as the enzymatic activity.
It is also important to comprehensively understand the target substrate proteins cross-linked by each TGase isozyme.
So far, we have identified peptide sequences containing Gln residues that are specifically used for crosslinking reaction by TGase in their isozyme-specific manner,
and have used these peptides and labeled primary amines that mimic Lys residues to investigate the level and localization of enzymetic activities,
as well as globally identify substrate protein for each TGase isozyme in various physiological (cell differentiation and skin formation) and pathological tissues (cell death, inflammation, and fibrosis in the liver, kidney, and lung).
Refernces for Highly Reactive Substrate Peptide
・TG2 (pepT26) and FXIII (F11): J Biol Chem. vol.281, 17699 (2006)
・TG1 (pepK5): FEBS J. vol. 275, 5667 (2008)
・TG3 (peE51): FEBS J. vol. 277, 3564 (2010)
・TG6 (pepY25): FEBS J. vol. 280, 1420 (2013)
・TG7 (pepZ3S): Arch Biophys Biochem. Vol. 537, 138 (2013)
We are going to analyze the in situ activity patterns for other specific peptide to each isozyme (FXIII, TG3, TG6, and TG7).
In situ Activity Data using FITC-labeled substrate eptides
In the figure, we would like to show the expression patterns of in situ
activities and the protein detected by immunostaining for TG1 and TG2.
In latter article (JHC 2013), we developed for the studies on mouse embyo
development.
The summarized table were indicated these data in each tissues.
References:
・J Cytochem Histochem. Vol. 59, 180 (2011)
・J Cytochem Histochem. Vol. 61, 793 (2013)
・Amino Acids. 49(3), 615-623 (2017)
Fig. 1
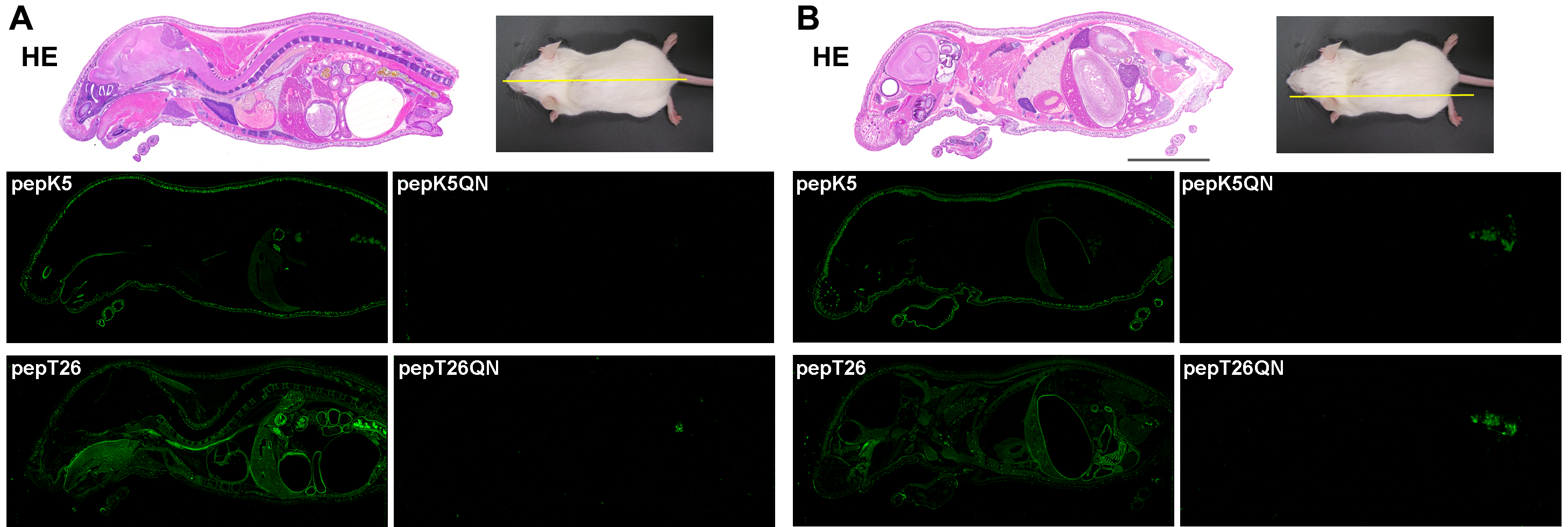
Fig 1. In situ activity patterns for TG1 and TG2 in whole-mouse sections
using FITC-pepK5 and T26
Whole-body sections were prepared and stained with hematoxylin and eosin (HE). The sections were reacted with 1 µM FITC-labeled peptides (pepK5, pepK5QN, pepT26, and pepT26QN) in the presence of CaCl2.
(A) Midline and (B) sideline. The bars indicate 1 cm.
Nonspecific signals in the pictures for the QN mutants may be due to excrement
in the bowel.
Fig. 2
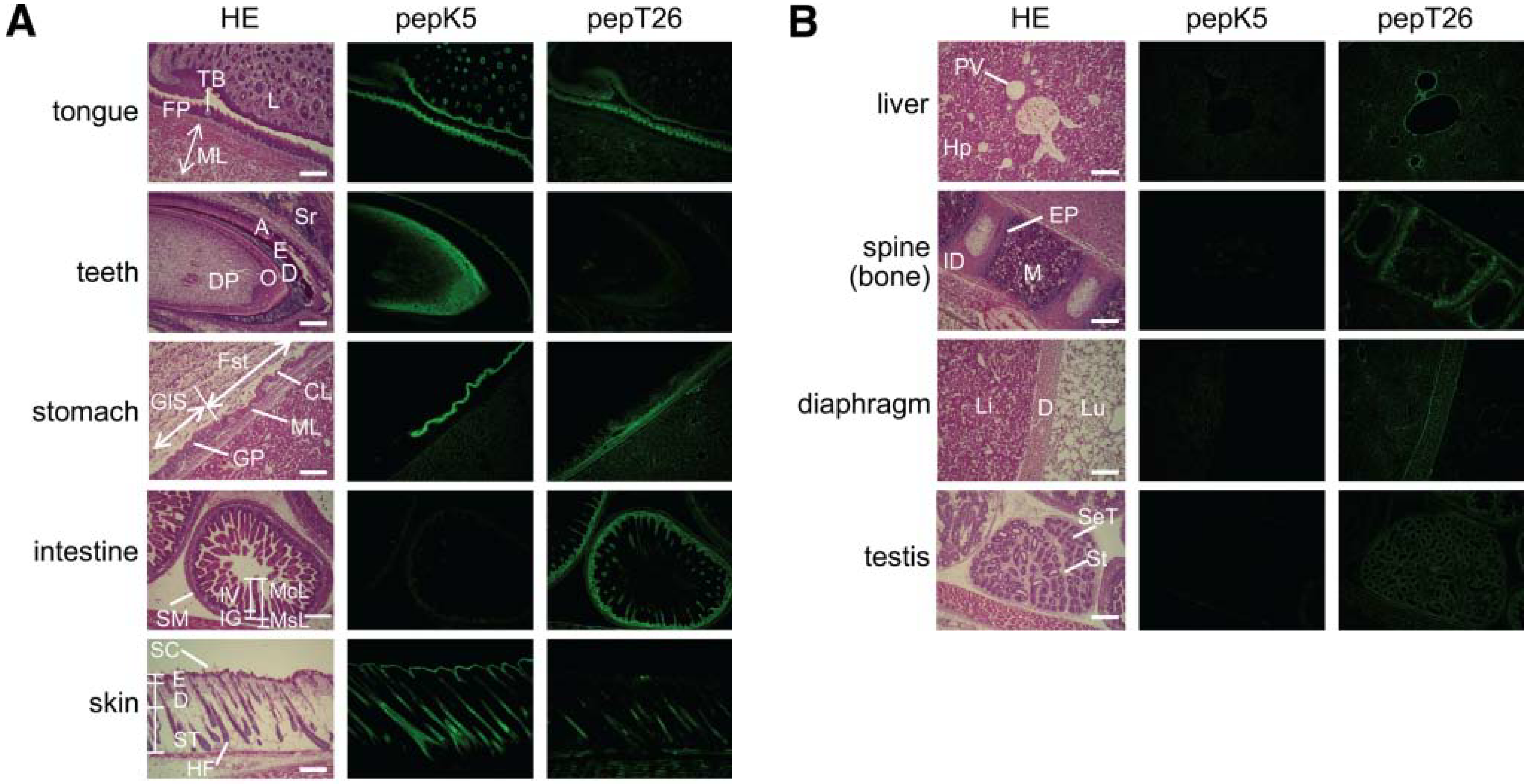
Fig 2. Tissue distribution of in situ active area of TG1 and TG2
Enlarged pictures of hematoxylin and eosin (HE) staining (left), fluorescence
imaging of FITC-pepK5 (center), and FITC-pepT26 (right) are shown for each
tissue.
(A) Tongue: FP, filiform papilla; ML, muscle layer; L, lip, TB, taste bud. Teeth: DP, dental papilla; O, odontoblast; D, dentin; E, enamel; A, ameloblast; Sr, sterate reticulum. Stomach: Fst, forestomach (stratified); GlS, Glandular stomach (monolayer); CL, cornified layer; GP, gastric pits; ML, muscle layer. Intestine: McL, mucosal layer; MsL, muscle layer; IV, intestinal villi; IG, intestinal glands; SM, seronous membrane. Skin: SC, skin cornified layer; E, epidermis; D, dermis; ST, subcutaneous tissue; HF, hair follicle.
(B) Liver: PV, interlobular portal vein; Hp, hepatocyte. Spine (bone):
M, bone marrow; ID, intervertebral disk; EP, epiphyseal plate. Diaphragm
(D): Li, liver; Lu, lung. Testis: St, stroma; SeT, semiferous cells.
The bars indicate 200 μm.
Fig. 3
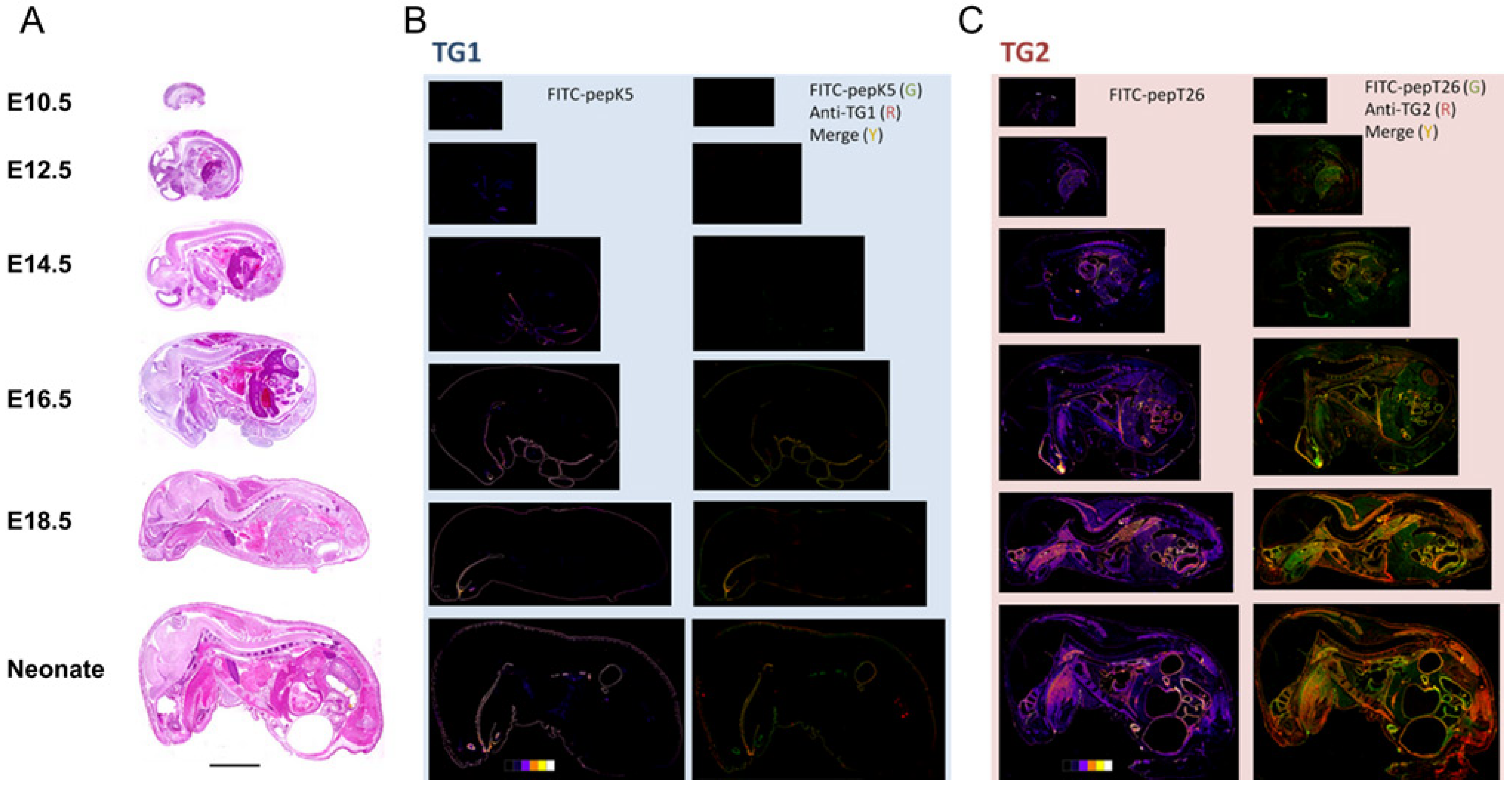
Fig 3. Simultaneous double staining for in situ enzyme activities and immune
detection
in whole-mouse body sections during embryonic developments
(A) Sections of embryos at various fetal development stages (E10.5, E12.5,
E14.5, E16.5, E18.5) and neonatal mice were stained with H&E staining.
(B) The sections at midline were subjected to in situ enzymatic activity
detection (FITC-pepK5) followed by immunostaining for TG1. Results in the
left panels show enzymatically active areas indicated by pseudo-color images.
Results in the right panels show merged areas (yellow) from the stained
areas for the enzymatic activity (green) and immunostaining (red).
(C) The sections at midline were subjected to in situ enzymatic activity
detection (FITC-pepT26) followed by immunostaining for TG2. Results are
in the same order as in (B). Bars = 0.5 cm.
Fig. 4
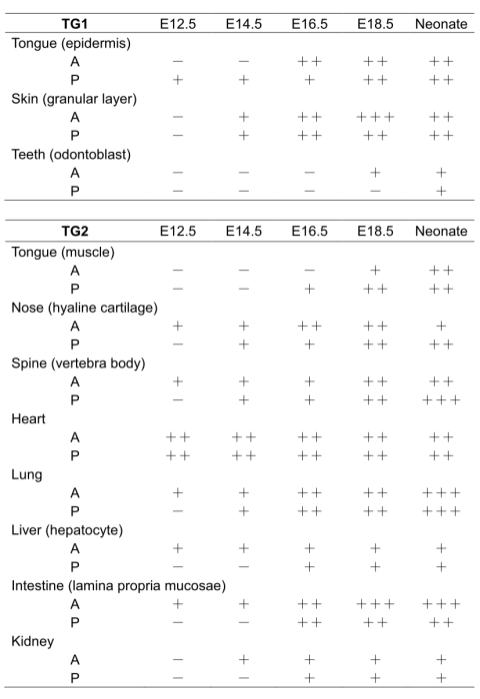
Fig 4. Summary for expressions of TG1 and TG2
by immunohistochemical analysis and in situ activity staining
TGase protein expression and activity levels: undetectable (-), low (+),
intermediate (++) and high (+++).
A and P indicate the levels of in situ enzymatic activity and immunologically
detected protein, respectively.
細胞死・細胞分化時のTG2の活性、基質タンパク質の解析
骨芽細胞の分化、胆汁酸による肝細胞障害、活性酸素による尿細管上皮細胞障害、線維化に伴う尿細管細胞の上皮間葉転換、マクロファージの極性化など種々の生命現象において、
TGaseが責任因子として働くことが分かっており、細胞レベルでのTGase活性の可視化や標的タンパク質の同定を進めている。
Candidate substrate proteins in differentiated osteoblast cell extracts.
Proteins identified by mass spectrometry were listed for each affinity-purified
samples incorporated biotin-pepF11KA (FXIII) or -pepT26 (TG2). Identified
proteins as possible substrates for FXIII and TG2 were listed here, respectively(Amino
Acids 2013).
F11KA-incorporated substrates (for FXIII substrates)
[Vimentin, Actin, Hsp71, Hsp90, Beta-actin-like protein 2]
78 kDa glucose-regulated protein, Ubiquitin carboxl-treminal hydrolase
47
Ras GTPase-activating-like protein IQGAP1, Hsp75, Glutamate dehydrogenase
1
ATP synthase subunit alpha, Tubulin alpha, Tubulin beta
T26-incorporated substrate (for TG2 substrates)
[Vimentin, Actin, Hsp71, Hsp90, Beta-actin-like protein 2]
Serpin H1, Hsp60, Lysozyme C-1, Endoplasmin
Collagen alpha-1(III) chain, Elongation factor 1-alpha 1
*Parenthesized these proteins as [] were identified as both TGase (FXIII and TG2) substrates.
References:
・Amino Acids 44, 209-214 (2013)
Detection and identification of possible substrate in differentiating cultured human keratinocytes
From the extract of differentiating cultured keratinocytes, the possible
lysine-donor substrates identified by incorporation of TG1-specific glutamine
donor substrate peptide were listed (BBRC 2016).Reproducible results were obtained
using different primary cultured cells.
Actin,
Annexin A1,
Annexin A2, Alfa-crystallin B,
Desmoplakin,
Epiplakin,
Fibronectin,
Junction plakoglobin, Heat shock protein β-1,
Hornein, Kallikrein-10
Galectin-7,
Involucrin, Plectin-1,
Plasminogen activator inhibitor 2,
S100A10,
S100A11
SPR1 (cornifin-A),
SPR2D, 40S ribosomal protein S13, 60S ribosomal proteins (L6, L13, L13, L15)
*The underlined proteins have been reported as components of CE.
References:
・Biochem Biophys Res Commun. Vol. 478, 343 (2016)
・FEBS J. Vol. 286, 2536 (2019)
・Anal Biochem. Vol. 603, 113606 (2020)
Fig. 1
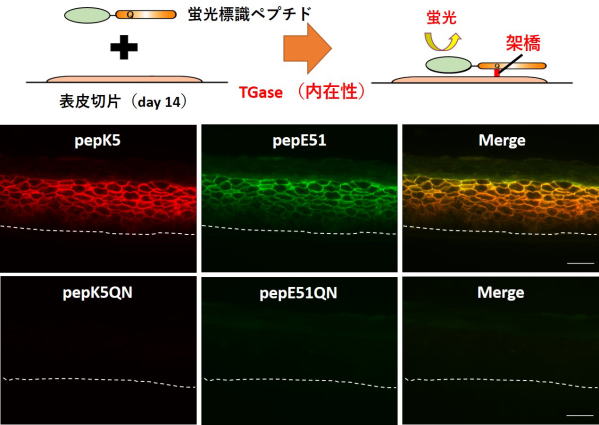
Fig 1. Visualization of in situ activities of TG1 and TG3 in the sections from the reconstructed epidermis.
The 3D cultured keratinocytes on day 14 on the filter were dissected and frozen without fixation. The sections containing cells and filter (beneath dotted membrane) were then prepared.
TAMRA-pepK5 or -pepK5QN (0.25 µM) and FITC-pepE51 or -pepE51QN (8 µM) were treated on the section, and their fluorescent signals were observed.
Reactions with both mutant peptides (QN) were carried out as a complete negative control. Reaction with labeled pepK5 and pepE51 detected both activities.
Fluorescent signals with red (TG1, left panel), green (TG3, center panel), and the merged images (right panel) were shown. The scale bar shows 50 µm.
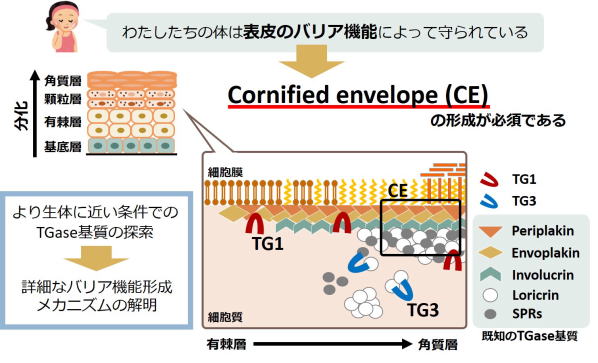
Fig 2. Maturation mechanism of cornified envelope for skin barrier.
TG1およびTG3の架橋活性が細胞膜表面の裏打ちタンパク質としてのCornified envelopeの形成を制御する。
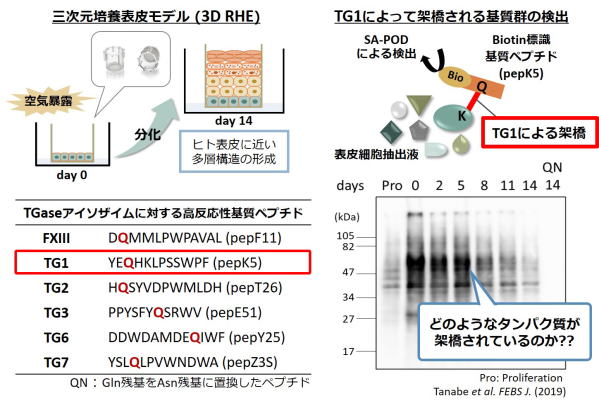
Fig 3. Detection of the possible substrates crosslinked by TG1 using substrate
peptide (pepK5).
表皮三次培養モデルにおいて、表示分化時に活性化するTG1により架橋される標的基質タンパク質を検出した。
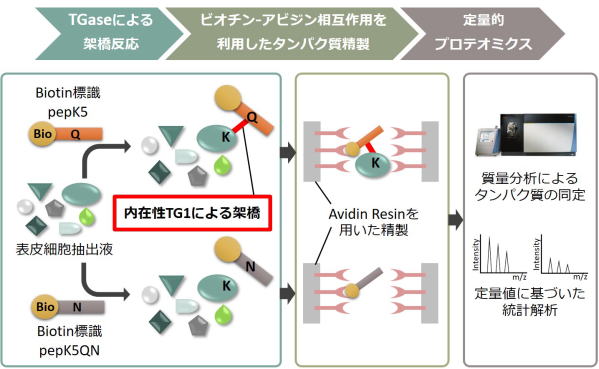
Fig 4. Global identification method of possible substrates crosslinked
by TG1.
ビオチン標識したTG1の基質ペプチド(pepK5)を表皮細胞の組織抽出液を反応させ、TG1による架橋反応によりビオチン化される標的基質タンパク質群をアフィニティーレジンにより精製し、質量分析によるプロテオーム解析により網羅的に同定する。
これにより、CE形成におけるTG1の役割を明らかにし、表皮形成時の細胞分化における制御機構を理解する。
Investigation of the molecular mechanism by which TGase involves in the
several diseases
(tissue injury, inflammation, and fibrosis) using animal models.
生理的な反応である血液の凝固、皮膚形成、死細胞の除去だけでなく、TGaseは多くの疾患(肝腎疾患、皮膚疾患、神経変性疾患、糖尿病、癌、血栓形成、自己免疫疾患など)発症の原因になります。
肝硬変・腎不全・肺線維症など各種臓器を対象として、組織の障害・炎症を伴う組織の硬化(線維化)に注目し、研究を行っています。
各種疾患動物モデル(肝線維化、アルコール性・非アルコール性脂肪性肝炎、糖尿病性腎症、腎線維化、急性腎障害、特発性肺線維症)を作製して 発症原因に繋がる分子メカニズムを解明してきました(Sci
Rep 2017/2018、FEBS J 2018、ABB 2018、Anal Biochem 2020)。
上記の疾患において機能未知の多くの候補基質タンパク質群を同定しており、今後は基質タンパク質の機能解析を進めることにより、 異常な架橋形成に起因する疾患の病態機序の解明や予防・治療法、疾患マーカーの開発などに繋げていきたいと考えています。
References:
・Sci Rep. Vol. 7, 45049 (2017) 下記 Fig. 1 and Fig. 2
・Sci Rep. Vol. 8, 7306 (2018) 下記 Fig. 3 and Fig. 4
・FEBS J. Vol. 285, 3056 (2018)
・Arch Biochem Biophys. Vol. 660, 11 (2018) 下記 Fig. 5
・Anal Biochem. Vol. 604, 113629 (2020)
Fig. 1
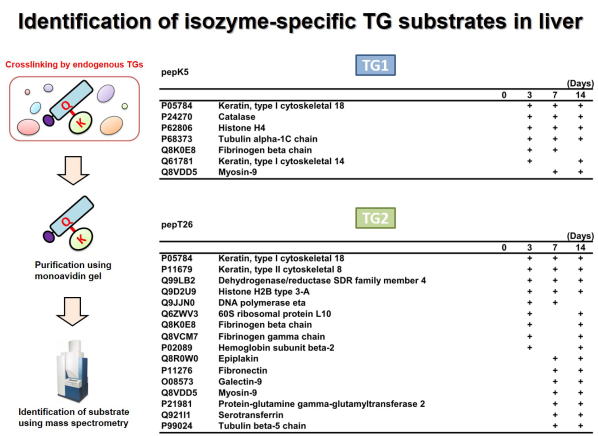
Fig 1. Global identification of possible Lys-substrates crosslinked by
TG1 and TG2 in liver fibrosis.
肝線維化においてTG1およびTG2により架橋されるLys側の基質タンパク質群の網羅的同定を行った。 ビオチン標識したTG1およびTG2の基質ペプチド(pepK5, pepT26)を胆管結紮処置により誘導した肝線維化の組織抽出液と反応させ、TGaseによる架橋反応によりビオチン化される標的基質タンパク質群をアフィニティーレジンにより精製し、質量分析により網羅的に同定した。 それぞれ、TG1およびTG2のLys残基側の候補基質として同定されたタンパク質の一部を示す。 (Sci Rep 2017)
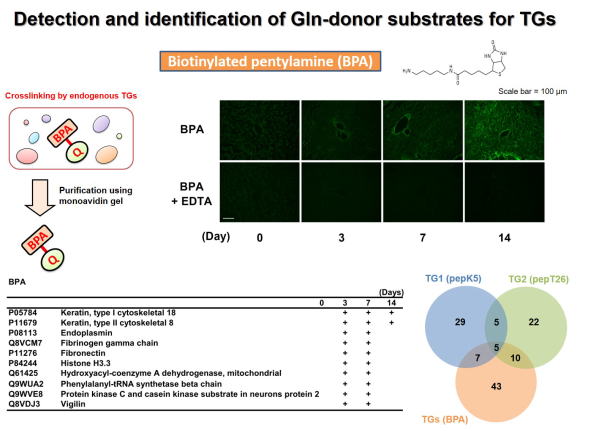
Fig 2. Global identification of possible Gln-substrates crosslinked by
TGase in liver fibrosis.
TGaseにより架橋されるGln側の基質タンパク質群の同定を行った。ビオチン標識したペンチルアミン(Lys残基を模倣)を肝線維化の組織抽出液と反応させ、TGaseによる架橋反応によりビオチン化される標的基質タンパク質群をアフィニティーレジンにより精製し、質量分析により網羅的に同定した。
BPAにはアイソザイム選択性がないため、TGaseのGln側基質タンパク質群が同定され、このうちの一部について示す。また、TG1およびTG2のLys残基側の架橋基質とTGaseファミリーのGln残基側の架橋基質として同定されたタンパク質の数をベン図により示した。
(Sci Rep 2017)
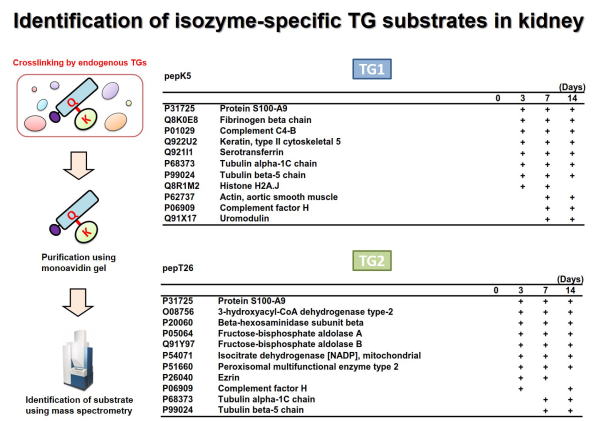
Fig 3. Global identification of possible Lys-substrates crosslinked by
TG1 and TG2 in kidney fibrosis.
腎線維化においてTG1およびTG2により架橋される基質タンパク質群の網羅的同定を行った。ビオチン標識したTG1およびTG2の基質ペプチド(pepK5,
pepT26)を尿管結紮処置により誘導した腎線維化の組織抽出液と反応させ、TGaseによる架橋反応によりビオチン化される標的基質タンパク質群をアフィニティーレジンにより精製し、質量分析により網羅的に同定した。
それぞれ、TG1およびTG2のLys残基側の候補基質として同定されたタンパク質の一部を示す。 (Sci Rep 2018)
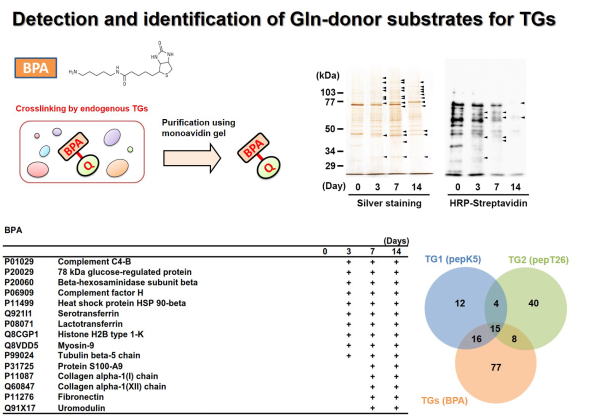
Fig 4. Global identification of possible Gln-substrates crosslinked by
TGase in kidney fibrosis.
TGaseにより架橋されるGln側の基質タンパク質群の同定を行った。ビオチン標識したペンチルアミン(Lys残基を模倣)を腎線維化の組織抽出液と反応させ、TGaseによる架橋反応によりビオチン化される標的基質タンパク質群をアフィニティーレジンにより精製し、質量分析により網羅的に同定した。 BPAにはアイソザイム選択性がないため、TGaseのGln側基質タンパク質群が同定され、このうちの一部について示す。また、TG1およびTG2のLys残基側の架橋基質とTGaseファミリーのGln残基側の架橋基質として同定されたタンパク質の数をベン図により示した。 (Sci Rep 2018)
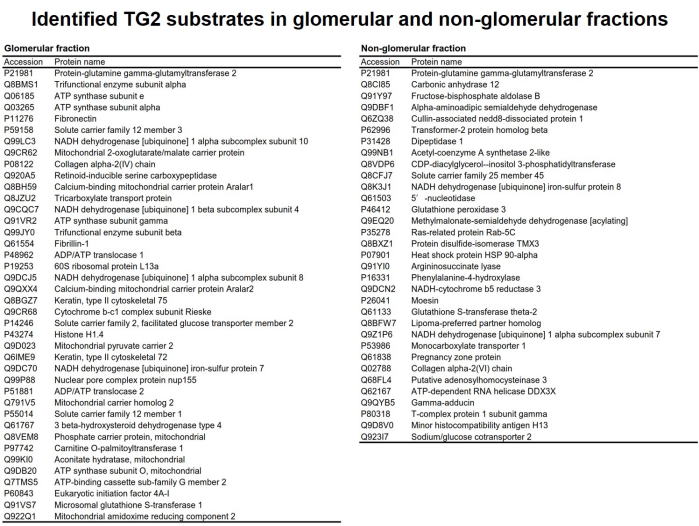
Fig 5. Potential TG2 substrates identified from glomerular and non-glomerular
fractions.
腎疾患におけるTG2の架橋反応の理解を深めるため、腎組織から糸球体およびそれ以外の細胞画分を抽出し、TG2に特異的な基質ペプチドpepT26およびpepT26QN(グルタミンをアスパラギンに置換したネガティブコントロール)を用いてTG2により架橋される基質タンパク質群の同定を行った。糸球体およびそれ以外の画分から調整した組織抽出液をビオチン標識pepT26QNと反応させ、非特異吸着性のタンパク質をプレクリーニングした後、ビオチン標識pepT26と反応させた。ビオチン化された基質タンパク質群をアフィニティーレジンにより精製し、質量分析によりノンラベル定量解析を行い、TG2の候補基質として同定されたタンパク質を示す。
(ABB 2018)
Hideki TATSUKAWA, PhD. Assistant Professor
E-mail: htatsukawa(at)ps.nagoya-u.ac.jp (replace (at) to @)
Kiyotaka HITOMI, PhD. Professor
Graduate School of Pharmaceutical Sciences, Nagoya University,
Tokai National Higher Education and Research System
E-mail: hitomi(at)ps.nagoya-u.ac.jp (replace (at) to @)












